44 fda health claims on food labels
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." Factual Food Labels: Health Claims - University of Texas at Austin Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims. In 1990, the Nutrition Labeling and Education Act allowed claims that related a specific food component (e.g., oats) to lowered disease risk (e.g., reduced cholesterol) to be printed on the label of a food product. For example, if a ...
Health Canada and FDA Regulatory Compliance Solutions Become Health Canada and FDA Compliant. We’ll help you meet regulatory requirements for NHP, Medical Devices, Food, OTC Drugs, VHP, Dietary Supplements, Cosmetics. Skip to the content. 1800-396-5144; info@qualitysmartsolutions.com; Amazon Seller Compliance. COVID-19 Compliance. CAN Compliance. Medical Devices (MDEL) NHP Licensing (NPN) Food/Label …

Fda health claims on food labels
Label Claims for Conventional Foods and Dietary Supplements 07.03.2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... Health claims on food labels - PubMed Health claims on food labels Food and drug law requires that the ingredients in most foods be disclosed on their labels, but until recently there was no requirement that nutrition information be provided. The Nutrition Labeling and Education Act of 1990 (NLEA), passed on November 8, 1990, mandated the Food and Drug Administrati … Enforcement Policy Statement on Food Advertising In addition to requiring nutrition information on virtually all food products, the NLEA directed FDA to standardize and limit the terms permitted on labels, and allows only FDA-approved nutrient content claims and health claims to appear on food labels.7 While the NLEA is designed in part to prevent deceptive and misleading claims on labels ...
Fda health claims on food labels. Health Claims on Food Labels | LegalMatch Do Health Claims Have to Be Authorized by the FDA? In short, yes. A health claim must be approved by the Food and Drug Administration ("FDA") before the manufacturer is allowed to put the health claim on one of their food products. In general, there are two ways in which a manufacturer can obtain FDA approval: 5 Understanding Food Labels and Health Claims - Maricopa Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease." Guidance for Industry: Food Labeling Guide | FDA You also can consult FDA's Industry Resources. Contact Us Office of Nutrition and Food Labeling, HFS-800 Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive... Health claims on food labels - Food labels - Canadian Food Inspection ... A health claim is any representation in labelling or advertising that states, suggests, or implies that a relationship exists between the consumption of a food and health. All aspects of food labels and advertisements contribute to the overall impression made by a food product, including health claims.
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if... Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims. Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
U.S. FDA Food Labeling Regulations - Top 5 Things to Know Labels cannot bear inappropriate claims. FDA has strict regulations for claims made on food labels. Some types of claims may be used as long as a product meets certain criteria, while other claims require FDA evaluation. There are three main types of claims that can be made on food labels: Health Claims, which characterize the relationship of ... Definitions - Health claims on food labels - Food labels - Canadian ... Health claim Any representation in labelling or advertising that states, suggests, or implies that a relationship exists between the consumption of a food and health. Laxation The normal softness and bulking of the stool resulting from such factors as increased undigested residue or bacterial mass, trapping of gases or water retention. Health Claims on Food Labels | Kaiser Permanente The health claims must be balanced and based on current, reliable scientific studies. And the claims must be approved by the U.S. Food and Drug Administration (FDA). Health claims may be statements like: "This food is a good source of calcium. Adequate intake of calcium may reduce the risk of osteoporosis." What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims)
FDA sued over health claims on food labels | Center for Science in the ... Two consumer groups sued the Food and Drug Administration (FDA) this week, charging that the agency broke the law when it decided to let food manufacturers tout unproven health claims on food labels. The suit, filed by Public Citizen and the Center for Science in the Public Interest (CSPI), says that current food labeling law requires health claims on food to be backed up by "significant ...
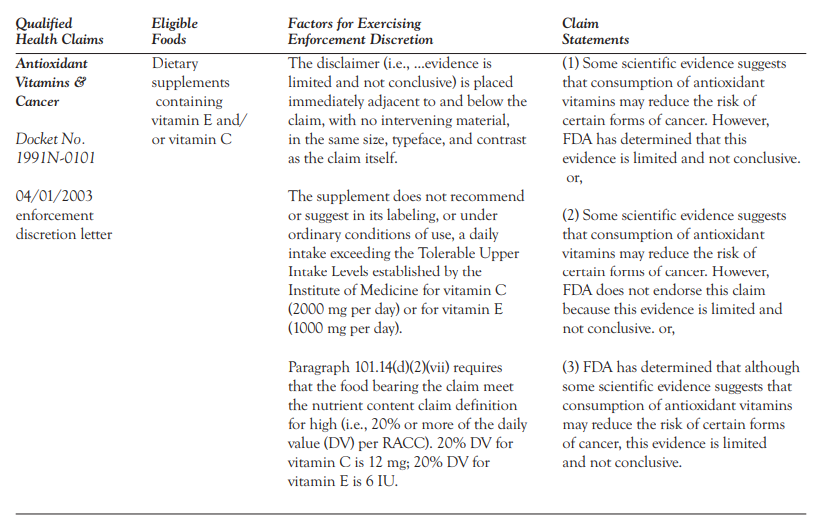
Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Label Claims for Food & Dietary Supplements | FDA 07.03.2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements...
Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
FDA finalizes qualified health claim for barley products May 18, 2006. The Food and Drug Administration announced May 19 that it has finalized a rule that allows foods containing whole-grain barley to claim that they reduce the risk of coronary heart disease. Specifically, whole barley and dry, milled barley products such as flakes, grits, flour, meal and barley meal, which provide at least 0.75 ...
Food Label Claims: What You Can and Can't Trust - WebMD The FDA is updating its definition for this claim. Until then, companies can make the "healthy" claim if the fats in their foods are mostly mono- and polyunsaturated fats. The healthy claim also...
FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Abstract The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA).
FDA: Labels Misleading on Major Food Brands - webmd.com The FDA today warned 17 food makers -- including POM, Gerber, and Nestle -- that their "misleading" product labels violate federal law. It's a new get-tough policy, FDA tells the food industry.
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...
Enforcement Policy Statement on Food Advertising In addition to requiring nutrition information on virtually all food products, the NLEA directed FDA to standardize and limit the terms permitted on labels, and allows only FDA-approved nutrient content claims and health claims to appear on food labels.7 While the NLEA is designed in part to prevent deceptive and misleading claims on labels ...
Health claims on food labels - PubMed Health claims on food labels Food and drug law requires that the ingredients in most foods be disclosed on their labels, but until recently there was no requirement that nutrition information be provided. The Nutrition Labeling and Education Act of 1990 (NLEA), passed on November 8, 1990, mandated the Food and Drug Administrati …
Label Claims for Conventional Foods and Dietary Supplements 07.03.2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...




















:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652082/healthy-choice.0.png)













Post a Comment for "44 fda health claims on food labels"