44 structure function claims on dietary supplement labels
Structure/Function Claims Basics - Dietary Supplement Experts Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of humans, or characterize the documented mechanism(s) of action by which a nutrient or dietary ingredient acts to maintain such structure or function. Importantly, they cannot be disease claims. Solved Question 21 Structure/function claims on a dietary - Chegg Transcribed image text: Question 21 Structure/function claims on a dietary supplement O must acknowledge that the FDA supports the claims that are misleading can lead to the product being taken off the market prove that the supplement can prevent a disease prove that the supplement can treat a disease O have to be approved, as done for drugs, before they can appear on product labels
Labeling Guidance: Making Structure/Function Claims - Lexology The FDA permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in ...

Structure function claims on dietary supplement labels
Understanding Dietary Supplement Claims | Consumer Healthcare ... - CHPA 1. Structure/function claims are the most common, permissible claim used for dietary supplements. DSHEA established special requirements for structure/function claims, including claims that are related to general well-being and nutrient deficiencies. Structure function claims can be easily confused with disease claims, which refer to a specific ... Structure Function Claims - Dietary Supplement Experts What laws and regulations govern structure/function claims? The Dietary Supplement Health and Education Act of 1994 (DSHEA) added section 403(r)(6) to the Federal Food, Drug, and Cosmetic Act (FD&C Act). This section of the law states that a dietary supplement may bear structure/function claims and provides their requirements. Structure/Function Claims | FDA The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of...
Structure function claims on dietary supplement labels. Dietary Supplements: An Advertising Guide for Industry In contrast to health claims, "structure/function" claims, within the broader category of "statements of nutritional support," refer to representations about a dietary supplement's effect on the structure or function of the body for maintenance of good health and nutrition. Structure/function claims are not subject to FDA pre-authorization. Dietary supplement - Wikipedia A dietary supplement is a manufactured product intended to supplement one's diet by taking a pill, capsule, tablet, powder, or liquid. A supplement can provide nutrients either extracted from food sources or that are synthetic in order to increase the quantity of their consumption. The class of nutrient compounds includes vitamins, minerals, fiber, fatty acids, and amino acids. Structure/Function Claim Notification for Dietary Supplements Office of Dietary Supplement Programs (HFS-810) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Contact the Office of... Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient...
Avoiding Misleading Claims On Your Supplement Product Label The market for dietary supplements in the United States grows by 7-10% every year. There is no question that taking a supplement can benefit your health; however, it is imperative that any health claims made on the box of a supplement be accurate and not overstated in any way. According to the statute and the Structure/Function Claims in Dietary Supplement Labeling: Not All of ... DSHEA and will explain why there are a significant number of "structure/function" claims that may be used in dietary supplement labeling without notification to FDA and without use of the DSHEA disclaimer in labeling. II. Provisions of DSHEA Concerning Structure/Function Claims DSHEA amended the Federal Food, Drug, and Cosmetic Act (FDCA)3 to add a Demystifying Dietary Supplement Claims part I: What is and What isn't a ... s/f claims (1) describe the role of a nutrient or dietary ingredient on the structure or function in humans (e.g., "calcium builds strong bones"); or (2) characterize the documented mechanism by which a nutrient or dietary ingredient acts to maintain such structure or function (e.g., "fiber maintains bowel regularity" or "antioxidants maintain … PDF Structure Function Claims: Substantiation Pitfalls A Structure Function Claim A statement describing the effect of a nutrient or dietary ingredient on a structure or function of the human body or characterizing the documented mechanism of such effect. These may appear on the labels of foods, dietary supplements, or drugs. • The definition of what a viable structure function claim varies though
structure function claims — FDA Reader — FDA Reader A Structure/Function Claim describe the role of a nutrient or ingredient on the structure or function of the human body. These may appear on the labels of foods, dietary supplements or drugs. Examples of a Structure/Function Claim: "Calcium builds strong bones" "Fiber maintains bowel regularity" PDF Permissible vs. Impermissible Structure/Function Claims for Dietary ... Structure/Function Claims for Dietary Supplements. 2 THE BASICS: ... the heart symbol on product label and labeling is an impermissible heart disease prevention claim.) 14 ... No more than thirty (30) days after a supplement bearing a structure/function claim is marketed, the manufacturer, packer, or distributor of the Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990... Label Claims for Food and Dietary Supplement - LBS RCS.COM The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary supplement labeling claims, claims of general well-being and claims related to a nutrient deficiency disease.
FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... The FDA permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or ...
FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in humans" or characterize the "documented mechanism" by which the nutrient acts to maintain such structure or function, but do not claim to "diagnose, …
FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure...
Statements For Dietary Supplements: Structure/Function Claims Review ... The FDA published final regulations defining the types of structure or function claims permitted on dietary supplement labels in 2000, 21 CFR 101.93: Certain Types of Statements for Dietary ...
Background Information: Dietary Supplements - Consumer The label of a dietary supplement or food product may contain one of three types of claims: a health claim, nutrient content claim, or structure/function claim. Health claims describe a relationship between a food, food component, or dietary supplement ingredient, and reducing risk of a disease or health-related condition.
''Structure/Function'' Claims in Dietary Supplement Labeling - ResearchGate Stephen H. McNamara Abstract Enactment of the Dietary Supplement Health and Education Act of 1994 (DSHEA) permitted manufacturers of dietary supplements to make structure/function'...
Challenge to Dietary Supplement Structure/Function Claims Preempted ... A structure/function claim "describes the role of a nutrient or dietary ingredient intended to affect the structure or function in humans" or "characterizes the documented mechanism by which a nutrient or dietary ingredient acts to maintain such structure or function." 21 USC 343 (r) (6); 21 CFR 101.93 (f).
Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA you must use a disclosure statement when you make a nutrient content claim and your food (including dietary supplements) contains one or more of the following nutrients in excess of the...
Structure/Function Claims - nutritionlabs.us Dietary Supplements. Structure/function claims have historically appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary ...
Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims
Structure/Function Claims | FDA The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of...
Structure Function Claims - Dietary Supplement Experts What laws and regulations govern structure/function claims? The Dietary Supplement Health and Education Act of 1994 (DSHEA) added section 403(r)(6) to the Federal Food, Drug, and Cosmetic Act (FD&C Act). This section of the law states that a dietary supplement may bear structure/function claims and provides their requirements.
Understanding Dietary Supplement Claims | Consumer Healthcare ... - CHPA 1. Structure/function claims are the most common, permissible claim used for dietary supplements. DSHEA established special requirements for structure/function claims, including claims that are related to general well-being and nutrient deficiencies. Structure function claims can be easily confused with disease claims, which refer to a specific ...










:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3650624/quakerlabel-shelf.0.jpg)







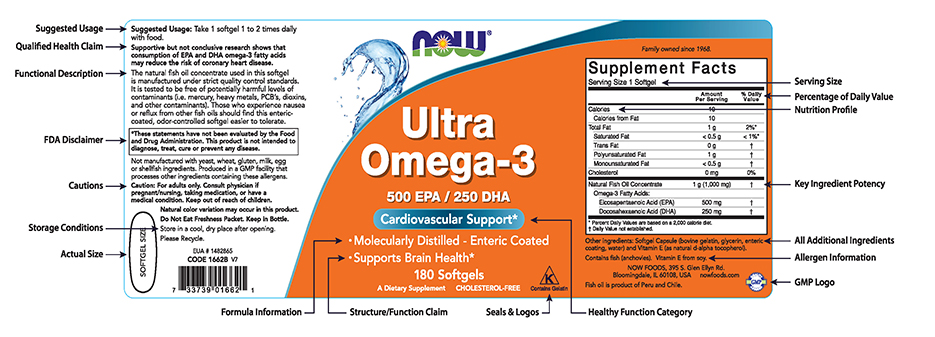









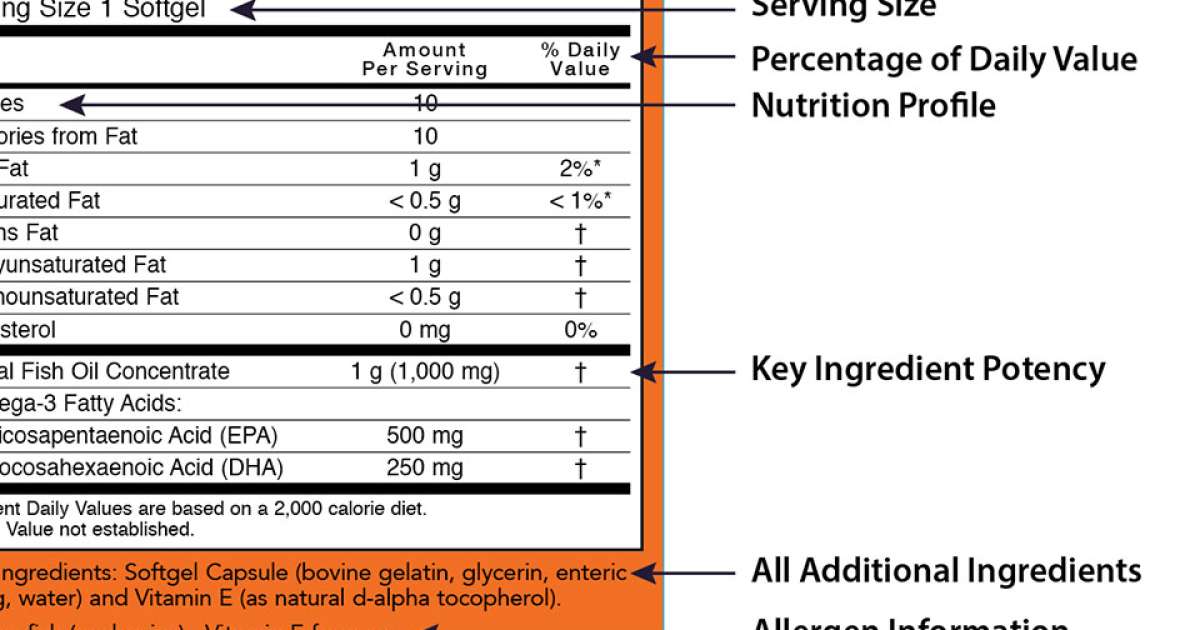







Post a Comment for "44 structure function claims on dietary supplement labels"